Welcome to the PLIETKER Group
Welcome to our dynamic organic chemistry group!
Here we work on an interface between natural products, drug design and catalysis to create molecules that matter!
The Group
In 2020, we moved from Stuttgart to the Dresden Technical University.
Our research interests include metal catalysis and total synthesis of natural products, as well as the development of biologically active compounds to pursue our journey from molecules to matter.
We see our lab as a place to grow and share innovative ideas and learn from each other's expertise.
Stay updated and check out our latest news:
Research
The main research areas within the Plietker-group are (A) new catalysts, (B) sustainable transition metal catalysis and (C) development and application of natural and drug syntheses in medicinal chemistry.
(A) Development of new catalysts:
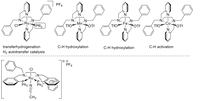
In recent years, the Plietker-group has carried out intensive development work in the field of the preparation of structurally defined transition metal complexes. As such novel Ru complexes using tetradentate (NNNN)- or (PNNP)-ligands which we specifically use in C-H activations, oxidations and reductions were prepared. In recent years, manganese and iron complexes have been increasingly included in the research portfolio as novel catalyst structures. The coordination behavior of the lighter transition metals is being determined and compared to ruthenium using NMR, X-ray spectroscopy (also using XAS, XES and, in the future, XPS methods), cyclovoltammetry (CV) or UV spectroscopy-cyclovoltammetry coupling and increasingly.
Another focus of current and future work in this area is the preparation of defined electron-rich Fe-NO complexes, which have a very interesting range of applications in catalysis due to the redox-active NO ligand. Understanding the coordination chemistry and structure-activity relationship of the new Fe-NO-complexes in organometallic reactions are of fundamental interest in order to develop new catalytic transformations.
(B) Development of novel catalytic transformations:
The development of organometallic catalysis in the Plietker-group is always benchmarked through application in the synthesis of complex functional molecules, e.g. in the field of natural product/active substance and material synthesis.
(i) Catalysis to increase resource efficiency:
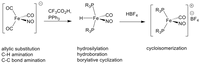
In the Plietker-group, the development of "sustainable catalysis" has been a core research area for many years (it was the subject of the habilitation thesis). The development of an atom-economic direct hydrovinylation of alkynes to di- and trienes using a defined Ru complex might serve as an early example (eq. (1)).
Specially designed (NNNN,P)-Ru complexes have been used for hydrogen auto transfer catalysis in which alcohols act as environmentally benign alkylating agents in C-C and C-N bond-forming reactions (eq. (2)).
Changing the donor atoms in the otherwise almost identical ligand backbone enables the efficient reduction of ketones by hydrogenation or transfer hydrogenation but also the reversible reduction of CO2 to formic acid (eq. (3)).
Very recently, a broadly applicable Ru-catalyzed chemoselective reduction of ketones, alkynes, or organonitro compounds was developed. The introduction of different additives to (Ph3P)3RuCl2 led to the in-situ formation of different Ru complexes, which show orthogonal chemoselectivities in the reduction of functional organic compounds in the presence of zinc and water (eq. (4)). With the help of this method, natural and active substances can be regio- and chemoselectively deuterated by zinc and D2O.
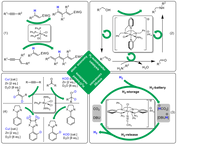
(ii) “Directed evolution” of Fe-NO complexes for use in catalysis:
The high specificity of biocatalysts is the result of directed evolution. The process of phylogenesis in the field of biocatalysis has led to a large number of highly specific biocatalysts arising from a manageable number of basic catalyst structures in the course of evolution. The Plietker-group would like to mimic this concept of phylogeny as part of a "directed evolution in the field of organometallic catalysis" in order to work on an archaea-type of catalyst, e.g. an Fe-NO complex, through systematic variation of catalytic conditions/ligands/additives in- build up highly specific reactivities in situ.
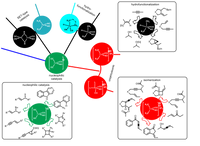
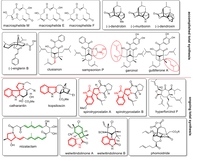
(C) Total synthesis
In the Plietker-group, a number of complex total syntheses of natural and active substances have been successfully completed in recent years. Many of these natural products exhibit interesting biological activities. However, it is the declared goal of total synthetic research to develop access natural substances in a few steps (max. 10 - 12 steps) and making substituent variations very easy on the molecular structure. With these basic requirements, the natural substances shown are to be further developed in future work in cooperation with groups from biology and medicine for specific indications. The fact that this can be achieved has been shown in recent years based on the systematic evaluation of the structure-activity relationships of polycyclic polyprenylated acylphloroglucinols (PPAP).
Publications and News
Teaching
Winter Term: "Stereochemie und asymmetrische Synthese", 'Mediziner Praktikum'
Summer Term: "Metallorganische Reaktionen in der Organischen Synthese", 'Grundlagen der Medizinalchemie', 'Syntheseplanung in der organischen Chemie'
Group meetings: Weekly Group Seminar and Denksport
.jpg/picture-200?_=180fa2ba148)